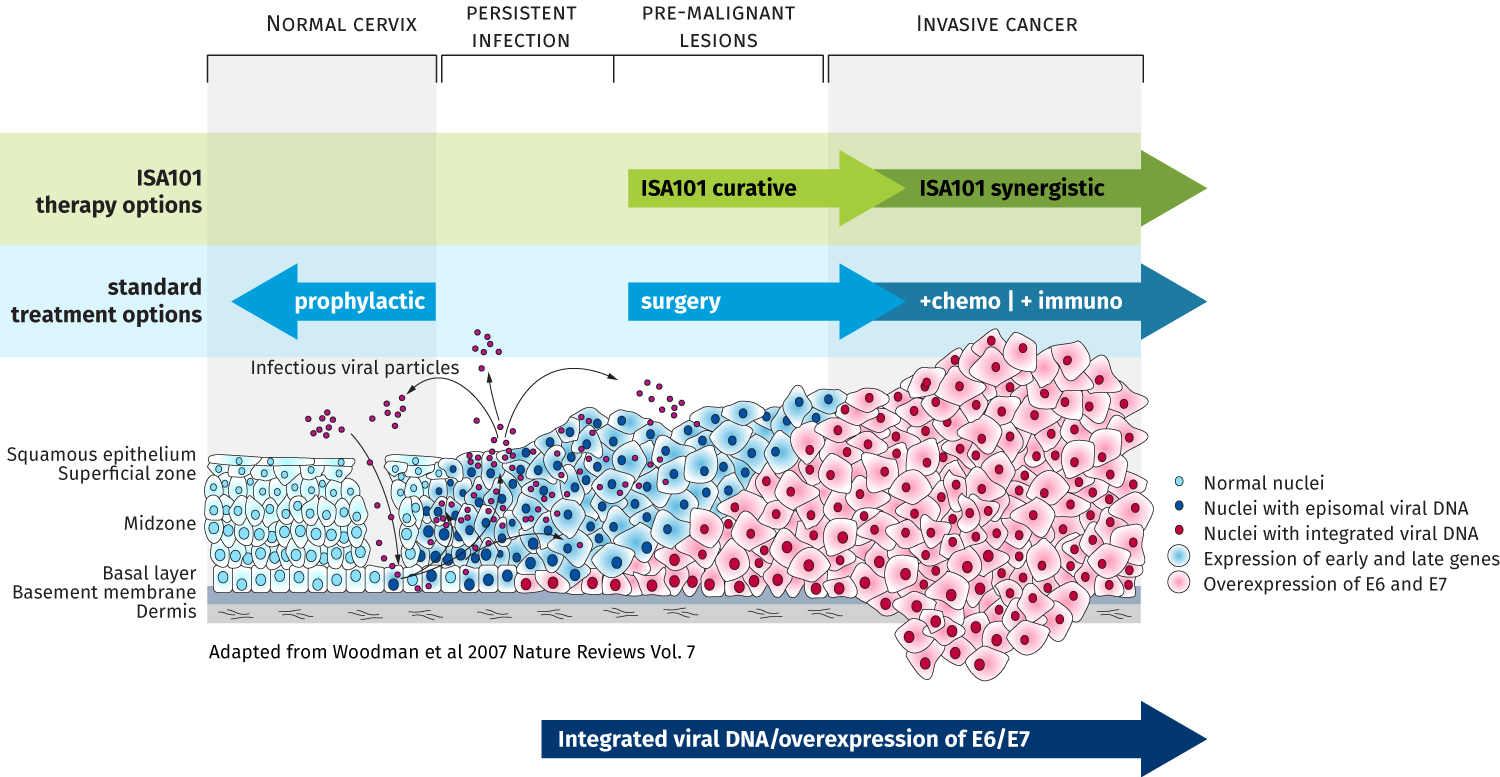
ISA101 consists of 12 synthetic long peptides (25 to 35 amino acids long) derived from the E6 and E7 oncogenic proteins of the HPV 16 virus, a strain responsible for over 50% of human cervical cancers and cervical intra-epithelial neoplasias, more than 85% of HPV-positive head and neck cancers, and similar substantial percentages of other premalignant and malignant HPV-induced lesions (such as VIN, vulvar cancer, AIN and anal cancer). It is currently administered either subcutaneously or intradermally.
Although most HPV16 infections cause no symptoms, are self-limited and cleared by a natural immune response, in some cases the virus becomes integrated into the DNA of cells, causing persistent infections that may lead to precancerous lesions and cancer.
Several prophylactic vaccines preventing HPV infections caused by a number of prevalent HPV types (2, 4, or 9 valent) are currently on the market. However, these vaccines are limited to preventing HPV infections and cannot cure established viral infections and associated lesions nor cancer once HPV is incorporated into the genomic DNA.